A) 1 m
B) 0 m
C) 12 m
D) 19 m
E) 97 m
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Thyroxine,an important hormone that controls the rate of metabolism in the body,can be isolated from the thyroid gland.If 0.454 g of thyroxine is dissolved in 10.0 g of benzene,the freezing point of the solution could be measured as 5.144°C.Pure benzene freezes at 5.444°C and has a value for the molal freezing-point-depression constant of Kf of 5.12°C/m.What is the approximate molar mass of thyroxine?
A) 775 g/mol
B) 7.75 g/mol
C) 7.75 × 105 g/mol
D) 42.7 g/mol
E) 11.3 g/mol
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fluids that mix with or dissolve in each other in all proportions are said to be _____.
A) immiscible fluids
B) miscible fluids
C) heterogeneous fluids
D) divergent fluids
E) suspension fluids
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of an aromatic hydrocarbon if 0.75 g of the compound depresses the freezing point of 123 g of benzene by 0.28°C? (Kf for benzene is 5.12°C/m.)
A) 43 g/mol
B) 150 g/mol
C) 110.00 g/mol 110
D) 2900 g/mol
E) 140 g/mol
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Osmotic pressure is
A) inversely proportional to mass fraction.
B) directly proportional to lattice energy.
C) inversely proportional to molality.
D) inversely proportional to mole fraction.
E) directly proportional to molarity.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a solution containing only one solute dissolved in a solvent,we can calculate the mole fraction of the solvent directly,given only
A) the molar mass of the solute.
B) the density of the solution.
C) the molar mass of the solvent.
D) the mole fraction of the solute.
E) the molarity of the solution.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following aqueous solutions would one expect to have the largest van't Hoff factor (i) ?
A) 0.500 m K2SO4
B) 0.500 m NaCl
C) 0.500 m C6H12O6 (glucose)
D) 0.050 m K2SO4
E) 0.050 m NaCl
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass percent of ethylene glycol (HOCH2CH2OH) in a solution of ethylene glycol in water that has a freezing point of -17.8°C? (Kf for water is 1.858°C/m.)
A) 99.8%
B) 37.2%
C) 59.4%
D) 64.7%
E) 9.58%
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of a 29.0% by mass glucose,C6H12O6,solution contains 60.0 g of glucose?
A) 52.2 g
B) 207 g
C) 17.4 g
D) 96.7 g
E) 60.0 g
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a 34.6% (by mass) solution is 129.0 mL.The density of the solution is 1.116 g/mL.What is the mass of solute in this solution?
A) 144 g
B) 49.8 g
C) 416 g
D) 40.0 g
E) 94 g
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 2.2-g sample of a small protein having a molecular weight of 42,000 g/mol is dissolved in 31.2 mL of water at 28°C.What is the osmotic pressure of the solution? (R = 0.0821 L · atm/(K · mol) )
A) 32 mmHg
B) 1300 mmHg
C) 2.9 mmHg
D) 18,000 mmHg
E) 0.041 mmHg
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a 24.0% (by mass) solution is 65.2 mL.The density of the solution is 1.072 g/mL.What is the mass of the solution?
A) 69.8 g
B) 61 g
C) 1670 g
D) 167 g
E) 17 g
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As the number of solute particles in a given volume of solution increases,
A) the boiling point will increase and the vapor pressure will increase.
B) the freezing point will decrease and the vapor pressure will decrease.
C) the freezing point will increase and the vapor pressure will increase.
D) the boiling point will decrease and the vapor pressure will decrease.
E) the osmotic pressure will decrease and the lattice energy will increase.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to _____,the partial pressure of solvent,,over a solution equals the vapor pressure of the pure solvent, 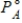 ,times the mole fraction of solvent,
,times the mole fraction of solvent, 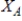 ,in the solution.
,in the solution.
A) Raoult's law
B) Benford's law
C) Dermott's law
D) Leibniz's law
E) Zipf's law
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is least soluble in water?
A) CH3CH2CH2NH2
B) CH3CH2CH2F
C) CH3CH(OH) CH3
D) CH3CH2COOH
E) CH3CH2NHCH3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of H2SO4 in a 38.2-cm3 sample of concentrated sulfuric acid that has a density of 1.84 g/cm3 and consists of 98.3% H2SO4?
A) 37.6 g
B) 69.1 g
C) 4.73 g
D) 1.81 g
E) 20.4 g
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which way of expressing concentration is used to relate the vapor pressure of a solution to the amount of nonvolatile solute dissolved in the solution?
A) mole fraction
B) molarity
C) osmotic pressure
D) mass percent
E) molality
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the formulas of the following solutes,which compound would have the smallest van't Hoff factor (i) ?
A) Ca(NO3) 2(aq)
B) K2SO4(aq)
C) Th(SO4) 2(aq)
D) Al2(SO4) 3(aq)
E) MgSO4(aq)
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Henry's Law constant is 0.034 mol/kg⋅bar and 0.0013 mol/kg⋅bar for CO2 and O2 respectively at 25°C.What pressure of O2 is required to achieve the same solubility as 0.368 bar of CO2?
A) 9.6 bar
B) 0.0 bar
C) ![]() bar
bar
D) ![]() bar
bar
E) 0.1 bar
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mole fraction of urea,CH4N2O,in an aqueous solution that is 37% urea by mass?
A) 0.15
B) 0.85
C) 0.37
D) 0.54
E) 0.66
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 119
Related Exams